
可编程核酸内切酶的出现彻底改变了基因组编辑技术,例如分子诊断和基因改造,对生命科学、生物安全、医疗和环境监测等产生了极其重要的影响。近几年,获得2019年诺贝尔生物学奖的基因编辑体系—(CRISPR)-Cas系统备受科学家和医疗企业的关注。该体系能对靶向核酸的特定位点进行编辑,从而引发相应物理特征变化,实现医学诊断与治疗。类似于CRISPR-Cas系统,Argonaute蛋白(Ago)同样是一类核酸介导的限制性核酸内切酶。该蛋白被提议为适用于一系列生物技术应用的基因编辑酶。相比于Cas蛋白分子量大、只能使用RNA作为介导核酸、需要PAM识别区等缺点,Ago蛋白具有分子量小、可使用RNA和DNA作为介导核酸、不需要PAM识别区、位点识别准确率高等优点。上述优势使Ago蛋白能够被应用于高通量的基因编辑和医学筛查。因此,研究Ago蛋白对核酸的剪切机制以及由此研发具有优异的核酸剪辑和结合活性的Ago蛋白,不仅是重要的科学问题,也具有重要的工程意义。
近日,beat365中文版官方网站beat365中文版官方网站/自然科学研究院/张江高等研究院洪亮课题组联合生命科学技术学院/微生物代谢国家重点实验室冯雁课题组利用多种实验技术和分子动力学模拟,发现嗜热Ago蛋白在其生理温度时采用了表面熔融的动态松散结构(与晶体结构不同),而常温Ago蛋白采用的是类似于晶体结构的构象。这种表面熔融的动态松散结构对嗜热Ago蛋白实现高效的DNA剪切功能具有关键作用。研究成果以“Loosely-packed Dynamical Structures with Partially-melted Surface Being the Key for Thermophilic Argonaute Proteins Achieving High DNA-cleavage Activity”为题发表于Nucleic Acids Research (IF: 19.160)。
本工作结合多种实验技术(SAXS,CD,DSF,nanoDSC和SDS-PAGE)和分子动力学模拟,研究了6种嗜热Ago蛋白在不同温度下的结构、动力学以及DNA剪切活性。我们发现:1. 相较于常温Ago蛋白,嗜热Ago蛋白在整体结构和活性中心处拥有更多的氢键、盐桥,从而导致嗜热Ago蛋白具有更高的热稳定性。与此同时,这种刚性的结构降低了Ago蛋白实现功能时所需的结构柔性,从而导致嗜热Ago蛋白在室温下DNA剪切活性很低;2. 在生理温度下,嗜热Ago蛋白采用了表面熔融的动态松散结构,这种结构介于晶体结构和蛋白完全解折叠结构。然而,常温Ago蛋白的功能态是其晶体结构;3.这种动态的、松散的结构能够赋予嗜热Ago蛋白极大的结构柔性,从而使其在生理温度下具有高效的DNA剪切活性。综上,本工作进一步拓展了原有蛋白质结构学中基于晶体结构的结构—功能关系。此外,本工作不仅对理解 Ago蛋白核酸剪辑的微观机制至关重要,而且为设计高活性嗜热蛋白提出了一种新思路:在室温下生物分子表面通过大量残基间的相互作用来限制其功能和保持结构完整性,但其中一部分相互作用在温度升高时会发生断裂,从而释放出足够的结构柔性以达到蛋白质的高酶活性。
更为重要的是,基于深度学习的AI算法在预测蛋白质分子的3D 结构方面已取得了巨大的成功,例如AlphaFold2。然而,作为用于训练机器学习模型的数据集大多是在常温甚至更低温度下确定的晶体结构,因此,预测的结构不可避免地只能模仿低温晶体结构。本工作表明,嗜热 Ago蛋白在其生理条件下通常呈现出部分熔融的松散堆积结构,这与常温或低温下的蛋白质晶体结构不同。我们的研究结果表明在今后的机器学习模型中需要考虑到蛋白质发挥生理功能时的状态与蛋白质晶体结构的差异性。
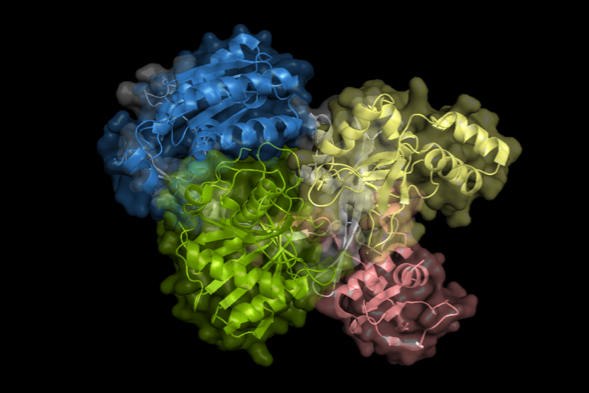
图:Ago蛋白三维结构示意图
洪亮教授为通讯作者,冯雁团队刘倩研究员为共同通讯作者。beat365中文版官方网站博士生郑力荣、生命科学技术学院硕士生陆慧、beat365中文版官方网站博士生昝冰和李松为共同第一作者。该工作得到了国家自然科学基金委、上海市科委、教委、上海人工智能国家实验室和张江高等研究院的支持,以及上海光源(SSRF)BL19U2谱仪科学家李娜研究员、beat365中文版官方网站分析测试中心和beat365中文版官方网站学生创新中心的技术支撑。
文章链接
https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkac565/6619476
Programmable endonucleases have revolutionized genome-editing technologies, such as molecular diagnostics and genetic modification, which are critical for life science, biosecurity, medical treatment, and environmental monitoring. In recent years, the clustered regularly interspaced short palindromic repeats (CRISPR)-Cas system has attracted great attention, which enabled target nucleic acids to be manipulated at a specific spot, thus leading to changes in physical traits and achieving medical diagnostics. Reminiscent of the CRISPR-Cas system, Argonaute proteins (Agos) are nucleic acid-guided endonucleases, which have been proposed to be suitable candidates for a range of biotechnological applications. Whereas Cas nucleases require the presence of a protospacer-adjacent motif (PAM) or protospacer flanking site (PFS), Agos are independent of such motifs. Furthermore, Agos can utilize DNA as guides that are much easier to produce and more stable during application than the RNA guides used by CRISPR-Cas. All the above advantages give Agos potential for easy-to-implement and high-throughput screenings in gene editing and medical diagnostics. Hence, studying of the microscopic mechanism underlying the DNA cleavage and design Ago with high efficiency of cleavage and binding is not only of scientifically important but also of great engineering significance. On June 30th, 2022, Nucleic Acids Research published a new edge research article entitled as “Loosely-packed Dynamical Structures with Partially-melted Surface Being the Key for Thermophilic Argonaute Proteins Achieving High DNA-cleavage Activity” by Prof. Liang Hong’s group (Institute of Natural Sciences/School of physics and astronomy/ Zhangjiang Institute for Advanced Study) and Prof. Yan Feng’ group (State Key Laboratory of Microbial Metabolism/School of Life Sciences and Biotechnology) . In the present work, we investigated the temperature dependence of structure, dynamics, and endonuclease activity of thermophilic pAgos (PfAgo and TtAgo) and their mesophilic counterpart CbAgo using various experimental and simulation techniques. We made the following discoveries: 1. The two studied thermophilic pAgos (PfAgo and TtAgo) possess stronger intramolecular interactions, including hydrogen bonds, salt bridges and hydrophobic interactions inside the protein core, globally over the entire protein and locally at the catalytic residues, than the mesophilic CbAgo. This renders thermal stability to thermophilic pAgos and strongly reduces their structural flexibility. 2. At physiological temperatures, PfAgo and TtAgo adopt a loosely-packed dynamical structure with a partially-melted surface which is an intermediate state between the crystalline structure and fully unfolded state, whereas CbAgo takes a compact crystalline structure at its optimal function temperature. 3. The partially-disrupted structure breaks many hydrogen bonds, salt bridges, and even some secondary structures on the surface of thermophilic pAgos to release the structural flexibility, leading to high cleavage activity under physiological conditions. This result is supported by the biochemical assays where we disrupted the structure of the two thermophilic pAgos by incubating them with urea and found significantly higher DNA cleavage activity compared to that of the pristine ones. Complementary kinetic analysis revealed that the partially-disrupted structure in thermophilic pAgos promotes its efficiency in cleaving the DNA without affecting its affinity for substrates. Our findings of thermophilic pAgos assuming loosely-packed dynamical structures with partially-melted surfaces under physiological conditions modify the existing structure-function relationship established based on the compact crystalline structures determined at moderate temperatures. It is not only crucial for understanding the microscopic mechanism of pAgos, but also suggests a smart design for the high-activity thermophilic proteins where a great amount of strong inter-residue interactions is required on the biomolecular surface at moderate temperatures to limit the function and preserve the structural integrity, but part of them is broken at the elevated desired temperatures to release sufficient structural flexibility to reach high activity.
More importantly, the deep-learning-based methods have recently achieved a great degree of success in predicting the 3D structure of protein molecules, e.g., alpha-fold. However, as the datasets used to train the machine-learning model are mostly crystalline structures determined at moderate temperatures or even lower, the predicted structures inevitably mimic the low-temperature crystal structures. The present work demonstrates that the thermophilic pAgos across a wide range of species generally assume loosely-packed partially-melted structures at their physiological conditions, which are different from the compact structures at moderate or low temperatures. Our findings call for an advanced version of the machine-learning model, which needs to consider the difference between the physiological condition where the protein functions and the one where the experimental structure is determined.
This is a collaborative work by Prof. Liang Hong and Prof. Yan Feng at SJTU. The first authors are Lirong Zheng, Hui Lu, Bing Zan and Song Li. The corresponding authors are Liang Hong and Qian Liu. The work was supported National Natural Science Foundation of China and Shanghai Municipal Science and Technology Major Project. The beam time was supported by Dr. Na Li from SSRF.
Link: https://academic.oup.com/nar/advance article/doi/10.1093/nar/gkac565/6619476




